Telomerase regulation in mice with the hmTert allele
The hmTert gene, containing the human 5’IR (23 kb), introns 2 (11 kb) and 6 (5.5 kb) (Fig. 1a) was highly expressed in embryonic stem cells and stringently repressed upon differentiation13. Terth/+ mice were obtained and mated with Tert+/- mice14, generating mice of Terth/-, Tert+/-, and Terth/+ genotypes. As shown in Fig. 1b, telomerase activity was readily detected in the majority of adult tissues in Tert+/- mice, yet it was present in a limited number of tissues in their Terth/- littermates. A direct comparison of mTert and hmTert mRNAs in Terthm/+ mice showed that mTert mRNA was expressed in most organs, whereas high hmTert mRNA expression was found only in thymus (Fig. 1c). Relatively low levels of hmTert mRNA were detected in testis and ovary, and very low levels in intestine and spleen. The hmTert mRNA expression pattern was similar to that of the hTERT mRNA in human tissues (Fig. 1d), suggesting that the postnatal regulation of the hmTert gene recapitulated that of hTERT in humans.
a Genomic maps of hTERT, mTert, and hmTert loci. Arrows indicate the directions of transcription. Vertical lines are exons; black and dark grey regions represent repetitive sequences, TEs, and VNTRs, respectively. Human and mouse 5’IR and introns 2 & 6 are labeled in blue and red, respectively. b Telomerase expression in tissues from Tert+/- and Terth/- littermates. Telomerase activities were determined by TRAP assay. 0.5 µg protein extracts from 4-month-old mice were used except for thymus (0.12 µg). +, h, and – refer to mTert, hmTert, and mTert-KO alleles, respectively. Organs from at least three different mice were examined and data from one representative mouse are shown here. c The expression of Tert mRNAs in adult mice. Tissues were collected from 4-month-old Terth/+ mice. hmTert and mTert mRNAs were distinguished by using primers overlapping with the silent mutations in exon 2 of the hmTert allele. P values, two tailed student’s t test, are shown. d hTERT mRNA expression in human tissues. Tert/TERT mRNA data were determined by qRT-PCR assay, normalized to 18S rRNA, and compared to those in Terth/+ ESCs or human ESC H1 cells (1.0). For c, d means and standard deviations (±SDs) of three technical repeats are shown. Repeated experiments showed similar results. Source data for panels b–d are provided as Source Data files.
During postnatal development, hmTert expression in testis, ovary, thymus, and intestine was more pronounced within the first two weeks of newborns and decreased with age in young mice (Supplementary Fig. 1a). Only thymus maintained significant hmTert expression. By comparison, mTert expression was high in most organs of newborns and decreased upon postnatal development, except for ovary, liver, and spleen, where high and low mTert mRNA levels were maintained in adults, respectively.
It was reported that hTERT expression progressively declined during T cell differentiation15 and T cells rapidly upregulated hTERT mRNA to support robust cell division and differentiation16. Consistently, resting mouse T cells expressed little hmTert mRNA and its level increase dramatically in CD4+ and CD8+ T cells following stimulation by CD3/CD28 antibodies for 48 and 72 h (Supplementary Fig. 1b). mTert RNA was readily detected in resting T cells and its level also increased during T cell activation. The increase of hmTert and mTert RNAs correlated with EdU incorporation, and thus cell proliferation, in T cells (Supplementary Fig. 1c).
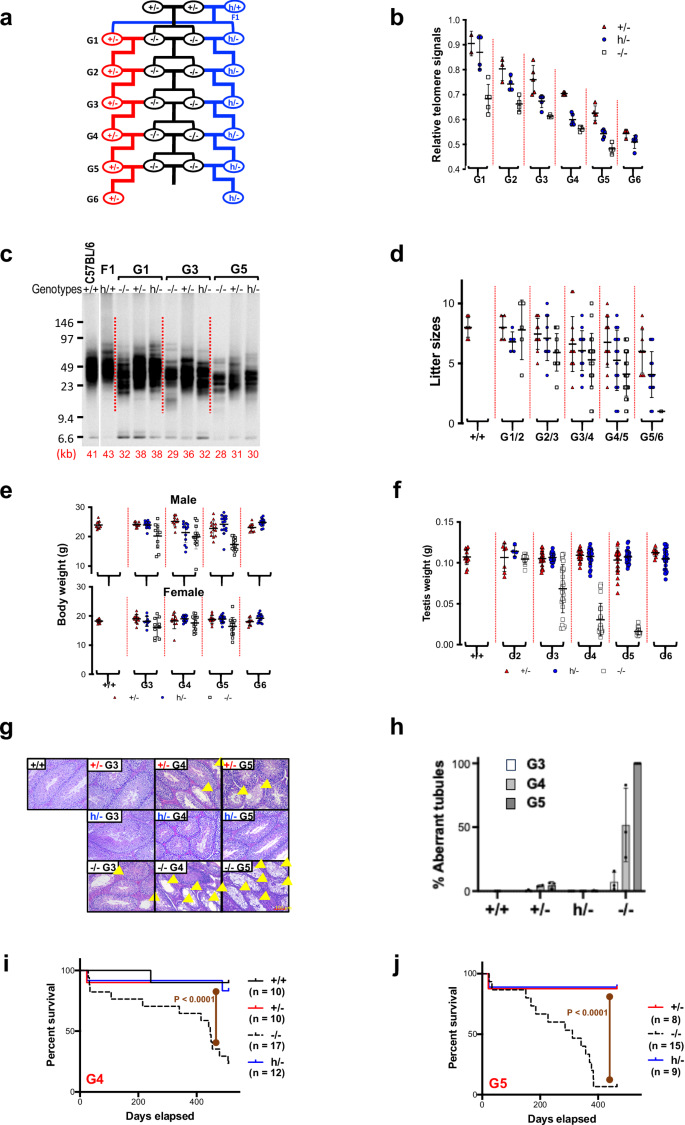
Telomere lengths in Tert+/-, Terth/-, and Tert-KO mice
C57BL/6 J mice have an average telomere length of approximately 50 kb. To reduce telomere length in mice with hmTert genes, Tert+/h F1 mice were crossbred to Tert+/- mice to produce Terth/- and Tert+/- offspring, which were then bred with successive generations of Tert-/- mice (Fig. 2a). Telomere length of these mice were examined by flow cytometry following fluorescence in situ hybridization (Flow-FISH) and telomere restriction fragment (TRF) analysis by Southern blotting (Fig. 2b, c). As the breeding of Tert-/- mice resulted in shortened telomeres, similar decreases in telomere length were observed in both Tert+/- and Terth/- mice over successive generations. In each generation, Terth/- mice had shorter telomeres on average compared to their Tert+/- counterparts. In G6 Terth/- mice, the average telomere length dropped to roughly 50% of the length in wildtype mice, or ~25 kb. The litter sizes of Tert+/-xTert-/- and Terth/-xTert-/- mattings declined over generations, while the litter sizes of homozygous Tert-/- incrosses decreased even more dramatically (Fig. 2d). In several attempts, G6 Tert-/- mice produced only one offspring which died prematurely, ultimately ending this breeding strategy. However, the reduction in telomere length in Tert+/- and Terth/- mice did not negatively impact their overall health and well-being, as evidence by their normal body weight in G6 mice (Fig. 2e).
a Breeding strategy. Telomere length of splenocytes from 2-month-old Tert+/-, Terth/-, and Tert-/- mice were determined by Flow-FISH (b) and telomere restriction fragment (TRF) analysis (c). b Telomere Flow-FISH. Telomere signals were detected by hybridization to FAM-(CCCTAA)3 oligonucleotide. Fluorescence signals were compared to that of wildtype C57BL/6 J mice (1.0). c TRF analysis. Splenocyte genomic DNAs were digested with HinfI and RsaI, followed by pulsed-field gel electrophoresis and Southern blotting. Positions of size markers are shown on the left (kb). d Litter sizes of breeding between Tert+/- and Tert-/- (red triangles), Terth/- and Tert-/- (blue circles), Tert-/- and Tert-/- (white squares) mice. e Body weight of male (upper) and female (lower) mice at 8-week of age. f Testis weight of mice at 10–15-week age. g H&E staining of seminiferous tubules in testes from Tert+/–, Terth/-, and Tert-/- mice. Yellow arrowheads indicate aberrant tubules. h Average percentages of aberrant seminiferous tubules in testes. +/+, n = 3; +/-: G3, n = 3; G4, n = 2, G5, n = 3; h/-: G3, n = 3, G4, n = 3, G5, n = 4; -/-: G3, n = 3, G4, n = 3, G5, n = 3. i, j, Survival curves of mice with mTert, hmTert, and mTert-KO alleles. Mice were bred as shown in panel a. Kaplan-Meier survival curves of G4 (i) and G5 (j) mice are shown. P-values of survival curve comparisons were calculated using logrank test. Each datapoint in panels b, d, e, f, & h represents one biological repeat (one animal). Means ± SDs are indicated. Source data for panels b–f and h–j are provided as Source Data files.
Maintaining testis homeostasis by the hmTert gene
Due to the impact of shorter telomeres on mouse fertility, we analyzed the testes of these mice. As illustrated in Fig. 2f–h, Tert-/- mice showed testicular atrophy as well as a progressive loss of germ cells in seminiferous tubules starting from G3 mice and worsening in G4 and G5 mice, as previously reported in telomerase deficient mice17. Tert+/- mice also exhibited a low level of testicular defects in G4 and G5, but such defects were absent in Terth/- mice. These data indicate that both mTert and hmTert genes help preserve germ cells in the testis, with hmTert showing a slight advantage in preventing germ cell loss.
Sustaining mouse lifespan by the hmTert gene
Previous studies have shown that telomere deficiency can impact mouse survival and lifespan18,19. Our results align with those findings, as shown in Fig. 2i, j, G4 and G5 Tert-/- mice had a significantly shortened lifespan, with median survival of approximately 440 and 320 days, respectively. However, the majority of wildtype Tert+/+, G4 and G5 Tert+/- and Terth/- mice lived past 500 days, indicating that the presence of a mTert or hmTert gene could sustain longevity even when telomeres were relatively short.
Rescuing telomere dysfunction in Tert-/- mice by the hmTert gene
Offspring inherit not only parents’ genotypes but also the lengths of their telomeres. G5 Tert-/- mice displayed severe telomere dysfunction, evidenced by tissue dystrophy, reduced body weight, and a shortened lifespan. The next generation, denoted as G6 Tert-/-m and G6 Tert-/-h, was generated by crossing G5 Tert-/- mice with G5 Tert+/- and Terth/- mice, respectively (Fig. 3a). Examination of telomere length through TRF and Flow-FISH analyses indicated that the average telomere lengths in G6 Terth/- and Tert+/- mice were comparable to, or slightly longer than, those of their G5 Tert-/- parents and G6 Tert-/- siblings (Fig. 3b and Supplementary Fig. 2a, b). However, despite these similarities, G6 Terth/- and Tert+/- mice exhibited significantly extended lifespans compared to their G5 Tert-/- mice (Fig. 3c). Notably, while all G5 Tert-/- mice died within 383 days, three out of 14 G6 Tert-/-h mice survived beyond the entire 460-day experimental period. This resilience could be attributed to the inheritance of short yet functional telomeres from their G5 Terth/- parents. Taken together, these findings suggest that the hmTert allele in Terth/- mice has the capacity to restore the shortest and likely most impaired telomeres, even in the context of their overall short average telomere length.
a Mouse breeding scheme. b TRF analysis of representative animals. Splenocyte genomic DNAs were digested with HinfI and RsaI, followed by pulsed-field gel electrophoresis and Southern blotting. c Kaplan-Meier survival curves of mice. P-values comparing indicated paired curves were determined using logrank tests. d Body weight of male (upper) and female (lower) mice at 8-week of age. e Testis weight of mice at 10−15-week age. Each datapoint in panels d & e represents a biological repeat. Means ± SDs are shown. P values shown are calculated using one-way Anova. Source data for panels b–e are provided as Source Data files.
Further examination of genotypes among G6 progeny indicated that fewer G6 Tert-/- offspring were born compared to their Terth/- siblings (Supplementary Fig. 2c), indicating that some Tert-/- offspring died during prenatal development. Although G6 Tert-/-m and G6 Tert-/-h mice had reduced body and testis weights compared to their Tert+/- and Terth/- littermates, respectively, their testes weighed significantly more than their G5 Tert-/- parents (Fig. 3d, e). In addition, G6 Tert-/-h mice had a slightly increased testis weight compared to G6 Tert-/-m mice, suggesting that the hmTert gene was functionally similar to, or somewhat better than, the mTert gene for rescuing testicular defects. Overall, G6 offspring showed better general health compared to their G5 Tert-/- parents, demonstrating the telomere function-restoring capacities of both the hmTert and mTert genes within a single generation.
hmTERT function in immune system
Previous studies have shown that hematopoietic cells’ proliferative capacity was compromised in telomerase-deficient mice, and human short telomere syndromes cause anemia, decreased erythropoiesis, and T cell immunodeficiency20,21,22. Consistent with earlier reports, peripheral blood from G5 Tert-/- mice exhibited a slight decrease in white blood cell (WBC) counts, a statistically significant reduction in red blood cell (RBC) counts, and normal platelet numbers (Fig. 4a). Among WBCs, there was a marked decrease of lymphocytes observed in G5 Tert-/- mice, accompanied by corresponding increases in neutrophils and monocytes. Both G6 Tert+/- and Terth/- mice had blood cell counts similar to wildtype mice. The lymphocyte and neutrophil cell percentages within WBCs of G6 Tert-/-h mice were between those of wildtype mice and their G5 Tert-/- parents. G5 Tert-/- mice had a dramatically reduced number of CD8+ T cells and a significant increase of the CD4/CD8 T cell ratio (Fig. 4b). Additionally, CD19+ B cells decreased in G5 Tert-/- mice. All these cell counts in G6 Tert+/- and Terth/- mice were restored to the levels found in wildtype mice. Further analyses of T and B cell counts in spleen and bone marrow revealed similar changes in G5 Tert-/- mice and Tert+/- and Terth/- offspring (Supplementary Fig. 3). In short, our data indicate that the hmTert gene effectively rescued blood cell defects in G5 Tert-/- mice within one generation.
a Whole blood cell counts in adult mice of 3–6 months by hematology analyses. b Lymphocyte counts in peripheral blood. Cells were stained using antibodies and analyzed by flow cytometry. Each datapoint represents one animal. Means ± SDs are indicated. P values shown are calculated using one-way Anova. Source data are provided as Source Data files.
hmTERT function in small intestine
The gastrointestinal tract is another high proliferation tissue that is affected by telomere dysfunction23. Depletion of the intestinal epithelial crypts and severe villus atrophy were observed in small intestines of older G5 Tert-/- (≥ 8 months), but not in G6 Tert+/- and Terth/- mice (Fig. 5a). The intestinal lesions probably contributed to the loss of body weight and overall poor health of Tert-/- animals due to decreased nutritional absorption.
a Histopathology of small intestines of adult mice of 8–10 months. The bar indicates 100 µm. b The expression of genes regulating cellular senescence and proliferation in small intestine. Each column represents an individual mouse. Relative mRNA levels were determined by qRT-PCR and normalized to 18S rRNA. Means ± SDs are shown. Source data are provided as a Source Data file.
The intestinal defects are likely a consequence of cell cycle inhibition and cellular senescence induced by telomere dysfunction. Therefore, the expression of genes involved in cell proliferation was examined. Tert mRNA was readily detected in the intestines of Tert+/+ and Tert+/- mice, but not in those of Terth/- or Tert-/- mice, confirming that the hmTert gene is strictly regulated in adult tissues (Fig. 5b). Markers of cell proliferation, Ki-67 and PCNA, and the cell cycle inhibitor p21 were found in the intestine of all genotypes. Senescence-associated genes, p16Ink4a and IL-6, were upregulated in G5 Tert-/- and G6 Tert-/-h mice, but not in any mice with mTert or hmTert genes. TNF-α, another pro-inflammatory cytokine secreted by senescent cells, appeared to be expressed in mouse intestines of all genotypes. Therefore, our data suggest that cellular senescence occurred in Tert-/- intestines with significant telomere dysfunction but was suppressed by the presence of the hmTert gene in this tissue.
Telomere shortening during intercrosses of Terth/- mice
Despite its restricted expression in adult tissues, the hmTert gene rescued telomere dysfunction in G5 Tert-/- mice with relatively short average telomeres of ~25 kb. Our next objective was to determine the telomere length setpoint influenced by the hmTert gene. To this end, G4 Terth/- mice were continuously intercrossed for 16 generations, from G4.1 to G4.16 (Fig. 6a). Using Flow-FISH, we monitored telomere length of Terth/- mice in splenocytes at each generation. As depicted in Fig. 6b, the average telomere length of Terth/- mice decreased from 60% to 18% of that observed in wildtype mice from G4 to G4.14, eventually stabilizing at 18–19% in the last three generations (G4.14 to G4.16). Throughout the breeding generations, both male and female Terth/- mice maintained body weights similar to those of wildtype mice (Fig. 6c). During this process, litter sizes varied, but were largely maintained (Fig. 6d). Male mice also maintained their testis weight (Fig. 6e). Figure 6f compares the average telomere length of all three genotypes in each generation, from G4.10 to G4.16. It demonstrates that Terth/h mice in general had longer telomere than their Terth/- siblings, and that Tert-/- mice consistently exhibited the shortest telomeres across generations. Overall, our data indicated that average mouse telomeres could be shorten to below 10 kb without affecting their overall health, at least at a young age, as long as they have the hmTert gene.
a Breeding strategy. Terth/- progeny from G4 Terth/- parents were intercrossed. b Telomere length as determined by Flow-FISH. Splenocytes from 2-month-old mice were used for the analyses. P value within the groups of G4.14, G4.15, and G4.16 was calculated using one-way ANOVA. c Body weight of 8-week-old male and female mice. d Litter sizes. e Testis weight of mice at 10–15-week age. f Flow-FISH comparing telomere lengths of Terth/h, Terth/-, and Tert-/- littermates. Each datapoint in panels b–f represents one animal. Means ± SDs are shown. n ≥ 3. g TRF analysis. Splenocyte genomic DNAs were digested with HinfI and RsaI, followed by 0.6% Agarose gel electrophoresis and Southern blotting. Sizes are indicated on the left (kb). MW, molecular weight marker. NHF (P11), passage 11 normal human foreskin fibroblasts. A longer exposure is shown in Supplementary Fig. 8a. h Genotype ratios of progeny in intercrosses at 7, 21, and 56 postnatal days. n = 21 ~ 93. Source data for panels b–h are provided as Source Data files.
Telomere length in later generations of mice was also verified using TRF analysis (Fig. 6g). Two types of telomeres were found in these mice: discrete bands of variable sizes and intensities between 15 and 20 kb, and shorter human-like telomere smears. The upper bands were sensitive to Bal-31 exonuclease digestion, indicating that they were indeed telomeres (Supplementary Fig. 4). The mean telomere lengths were 7–9 kb in G4.14–G4.16 Terth/h mice and 6–8 kb in the Terth/- mice (Supplementary Table 1). In a G4.14 Tert-/- mouse, the telomere smear was much less apparent. Figure 6h shows that, from G4.2 to G4.12, approximately 50% of total born mice were of heterozygous Terth/- genotype, while homozygous Terth/h and Tert-/- mice each accounted for about 25% of total progeny, following Mendelian genetics. However, there was a sharp decline in the numbers of Tert-/- mice born from G4.13 to G4.16. The few Tert-/- mice that were born were small and die at young ages. These data indicated that short telomeres in late-generation Tert-/- embryos could no longer sustain mouse development.
Maintaining stable human-like telomeres in homozygous Terth/h mice
To assess the stability of short telomeres in mice with the hmTert genes, homozygous Terth/h offspring from G4, G4.8, and G4.14 were incrossed for 13, 9, and 2 generations, respectively (Fig. 7a). Average telomere lengths in their progeny were measured by Flow-FISH. The results, depicted in Fig. 7b, revealed a gradual decrease in telomere length across successive generations. In G4 Terth/h mice, telomeres decreased from 60% to 30%, while in G4.8 Terth/h mice, the decline went from 34% to 24%. G4.14 Terth/h mice exhibited telomere lengths approximately 24% of the wildtype, and their offspring maintained telomeres at 22–23% of wildtype length during two successive generations of incrossing. These findings indicate that telomere length in Terth/h mice stabilized at a shortened but consistent ranges of 21–24% of wildtype mice, equivalent to an average telomere length of 10–12 kb, similar to reported leukocyte telomere lengths of 9.5 ± 0.7 kb and 10-11 kb in newborn humans2,24. Additionally, TRF analysis confirmed the presence of variable discrete telomere bands between 15–20 kb and a human-like telomere smear (Fig. 7c). For G4.8(g-i) and G4.14(a–b) mice, the average lengths of telomere smears were approximately 10 kb. Regardless of having shortened telomeres, these mice exhibited good health, as demonstrated by stable body weight, litter sizes, and testis weight (Fig. 7d–f).
a Breeding schemes. Terth/h progeny from G4, G4.8, and G4.14 Terth/- parents were successively incrossed. b Relative telomere signals. Telomere signals were determined by Flow-FISH and normalized to that of wildtype C57BL/6 J mice (50 kb). n ≥ 3. c TRF analysis. A longer exposure is shown in Supplementary Fig. 8b. d Body weight. e Litter sizes. f Testis weight. Each data point represents one animal. Means ± SDs are shown in panels b, & d–f. Source data for panels b–f are provided as Source Data files.
To further determine the health status of Terth/h mice with human-like telomeres, we performed hematology analysis on peripheral blood samples from G4.8 g, h, and i mice aged 2–3 months. As shown in Supplementary Fig. 5, the result indicated normal red blood cell counts, hemoglobin, and hematocrit in all groups of mice. Although the average WBC count in G4.8 h was lower than those of G4.8 g, G4.8i, and wildtype mice, it still fell within the normal WBC range of physiological data for C57BL/6 J mice published by the Jackson Laboratory25. Within WBC populations, all groups of mice displayed normal percentages of lymphocytes, monocytes, and neutrophils, further supporting the notion that Terth/h mice with human-like telomeres maintain good health at early stages of their life.
Comparing hmTert and mTert gene functions
In order to directly compare the functions of the hmTert and mTert genes and establish a another line of Terth/h mice, we conducted intercrosses using G6 Terth/- and Tert+/- mice (Fig. 8a). Consistent with our previous findings, telomere length in G6 Terth/- mice decreased from 55% to 18–19% of wildtype telomere length in C57BL/6 J mice across 12 successive intercrosses (Fig. 8b). In contrast, the average telomere lengths in G6 Tert+/- mice remained stable at 55% of wildtype telomere length over eight intercrosses. This result aligned with a previous study in which Tert+/- mice were intercrossed for 17 generations, stabilizing their average telomere length at approximately 50% of wildtype telomeres26. Importantly, G6 Terth/- mice exhibited consistent litter sizes and development with appropriate body and testis weights across the entire breeding process (Fig. 8c–e). Therefore, our data indicate that the Tert loci play a crucial role in regulating telomere length homeostasis, and the hmTert gene genetically determines short telomeres in mice.
a Breeding strategy. G6 Tert+/- and Terth/- mice from Fig. 2a were independently intercrossed. b Relative telomere signals as determined by Flow-FISH and normalized to that of wildtype C57BL/6 J mice. c, Body weight. d Litter sizes. e Testis weight. Each data point represents one animal. Means ± SDs are shown. Source data for panels b–e are provided as Source Data files.
Hematopoiesis following bone marrow ablation
A major problem in patients with telomere biology disorders are hematopoietic defects, manifested as decreased marrow reserves and increased sensitivities to cytotoxic agents27. Here, we examined the responses of Terth/h mice with short telomeres (G4.8i) to the chemotherapeutic agent 5-fluorouracil, or 5-FU. The numbers of white blood cells, red blood cells, and lymphocytes in the peripheral bood of both C57BL/6 J (Tert+/+) and Terth/h mice decreased significantly on days 6 and 11 post 5-FU injection (Supplementary Fig. 6). The numbers of platelets in both experimental groups also declined on day 6 but increased on day 11. The recovery of platelet numbers in Terth/h mice appeared to be slightly slower compared to those in wildtype mice. Therefore, the data showed that the ability to sustain and recover from 5-FU-induced damage in the hematopoietic system was not significantly impacted by short telomeres in Terth/h mice.
Decreased in vivo cell proliferation capacity in Terth/h mice
Ulcerative colitis is an inflammatory disease associated with telomere shortening and accelerated colon aging in human patients28. G4.8 h Terth/h mice remained in good health, and their gastrointestinal tracts appeared normal. As depicted in Fig. 9d,e, cellular proliferation, assessed by EdU incorporation, in the colons of G4.8 h Terth/h mice were similar to that wildtype mice. To evaluate tissue renewal capacity under pathological conditions, mice were subjected to a 6-day dextran sodium sulfate (DSS) treatment to induce conditions akin to ulcerative colitis29,30 (Fig. 9a). DSS treatment led to similar colon shortening and spleen enlargement in both Tert+/+ and Terth/h mice, indicating DSS-induced comparable inflammatory responses in both groups (Fig. 9b, c). The toxicity of DSS to colonic epithelial cells triggered a regenerative response upon toxin removal29. In Terth/h mice, an average of about 2 EdU-positive cells per crypt cross-section were observed, significantly fewer than the average of 7 EdU-labeled cells per crypt cross-section in wildtype mice (Fig. 9d, e). These results suggested that, while Terth/h mice with human-like short telomeres maintained tissue homeostasis during postnatal development and adulthood under normal physiological conditions, tissue renewal was more limited under pathological conditions due to their short telomeres and absence of telomerase activity.
a Experimental strategy. 7–8-month-old Tert+/+ (wildtype C57BL/6 J) and Terth/h (G4.8 h) mice were given drinking water with or without 3% DSS for 6 days, followed by 1 day of pure drinking water. Intraperitoneal EdU injection was performed 2 h before tissue collection. b Representative images of colons and spleens following DSS treatment. c Spleen weight. Spleen weight was normalized to the body weight of each mouse. Each data point represents one animal. n = 4. d EdU staining of colon crypt sections. Colon tissues were labeled with anti-EdU (white) and E-cadherin (green) antibodies, as well as Hoechst dye for nuclear staining (blue). Scale bar represents 100 µm. Representative images are shown. e Quantification of EdU-positive cells. Each datapoint in panel e represents the average number of EdU-positive cells per colon crypt in 30 crypts from one animal. n = 6. Means ± SDs are shown in panels c, e. P values, calculated using two-way Anova, are shown. Source data for panels c and e are provided as Source Data files.
Telomere integrity in mouse cells with human-like telomeres
To assess telomere integrity in somatic cells from mice with human-like short telomeres, mouse embryo fibroblasts (MEFs) were prepared from individual embryos of G4.16 Terth/- intercrosses. To minimize telomere damages, MEFs were culture in 3% oxygen31. Telomere length in passage 4 MEFs was measured using telomere fluorescence in situ hybridization (FISH) analysis. As shown in Supplementary Fig. 7a, both Terth/h and Terth/- MEFs exhibited lower telomere fluorescence compared to wildtype (Tert+/+) MEFs. Quantitative measurements revealed that the average telomere fluorescence signals of Terth/h and Terth/- MEFs were approximately 18% and 15% of those in wildtype MEFs, respectively (Supplementary Fig. 7b). These MEFs were stained with antibodies against TRF2 and γH2A.X. Damaged telomeres were identified as telomere dysfunction-induced foci (TIFs) through the colocalization of TRF2 and γH2A.X staining. As shown in Supplementary Fig. 7, panels c and d, Terth/h and wildtype MEFs had similar numbers of TIFs. However, TIF numbers were elevated in Terth/- MEFs and further increased in Tert-/- MEFs. These results indicate that while short telomeres in Terth/h mice are functional, telomere dysfunction is present in cells from Terth/- and Tert-/- mice.